The production process of lithium carbonate from lepidolite is introduced. Lithium carbonate is an important raw material for new energy batteries. With the formulation of the new energy development plan, lithium battery new energy has become a key support for the development of energy industry.
Lithium resources are rich, but the mining volume is only 5% of the world total. In the final analysis, it is due to the lack of process and technology. In recent years, with the progress of science and technology, more and more attention has been paid to the exploitation and application of lithium resources. Today, we will introduce the production process of lithium carbonate from lower lepidolite:
1. Comprehensive utilization of brine. After extracting barium chloride from brine, the lithium containing material solution is added with soda ash to remove calcium and magnesium ions in the material solution, acidified with hydrochloric acid, evaporated to remove sodium chloride, and then iron is removed. Then, excessive soda ash is added to precipitate lithium carbonate, washed, centrifuged, and dried to obtain lithium carbonate finished products.
2. Lime sintering method. Spodumene concentrate (generally containing 6% lithium oxide) and limestone shall be proportioned according to 1: (2.5~3) weight ratio. Mix and grind, sinter at 1150~1250 C to generate lithium aluminate and calcium silicate, grind and crush them by wet grinding, leach lithium hydroxide with washing solution, settle and filter, return the filter residue or wash and remove the residue, evaporate and concentrate the leaching solution, then add sodium carbonate to generate lithium carbonate, centrifuge and dry to obtain finished lithium carbonate.
Compared with the extraction of lithium by sulfuric acid method, the extraction of lithium from spodumene by limestone sintering method has the advantages of simple process, no consumption of valuable chemical raw materials, and widely used lithium hydroxide monohydrate.
3. Using lithium hydroxide and carbon dioxide as raw materials, high-purity lithium carbonate can be prepared. Lithium sulfate and sodium carbonate can also be used as reactants, but lithium carbonate is easily soluble in other salt solutions, so the yield is not very high, generally about 75%, and the product also contains a small amount of lithium sulfate.
4. Sulphuric acid method. The fused spodumene is reacted with sulfuric acid, purified and then reacted with sodium carbonate.
5. Lime method. The calcined spodumene is reacted with lime milk, purified and then reacted with sodium carbonate.
6. By product method. It is extracted from the mother liquor containing lithium after preparing barium chloride from well brine.
7. Take industrial lithium hydroxide as raw material, heat water to dissolve it, filter out the insoluble matter, inject clean carbon dioxide gas into the filtrate while it is hot until no precipitation is generated, filter while it is hot, shake it dry, wash it with hot distilled water until it is qualified, and then dry it. Dissolve industrial lithium carbonate in cold water, filter, boil the filtrate, stop heating, filter while hot, wash with hot water, shake dry and dry, and then prepare reagent lithium carbonate.
The above-mentioned technological process for lithium carbonate production from lepidolite is to prepare lithium carbonate finished products from lepidolite and spodumene. No matter which process is used, lepidolite or spodumene needs to be ground into fine powder by a grinder before going to the next process.
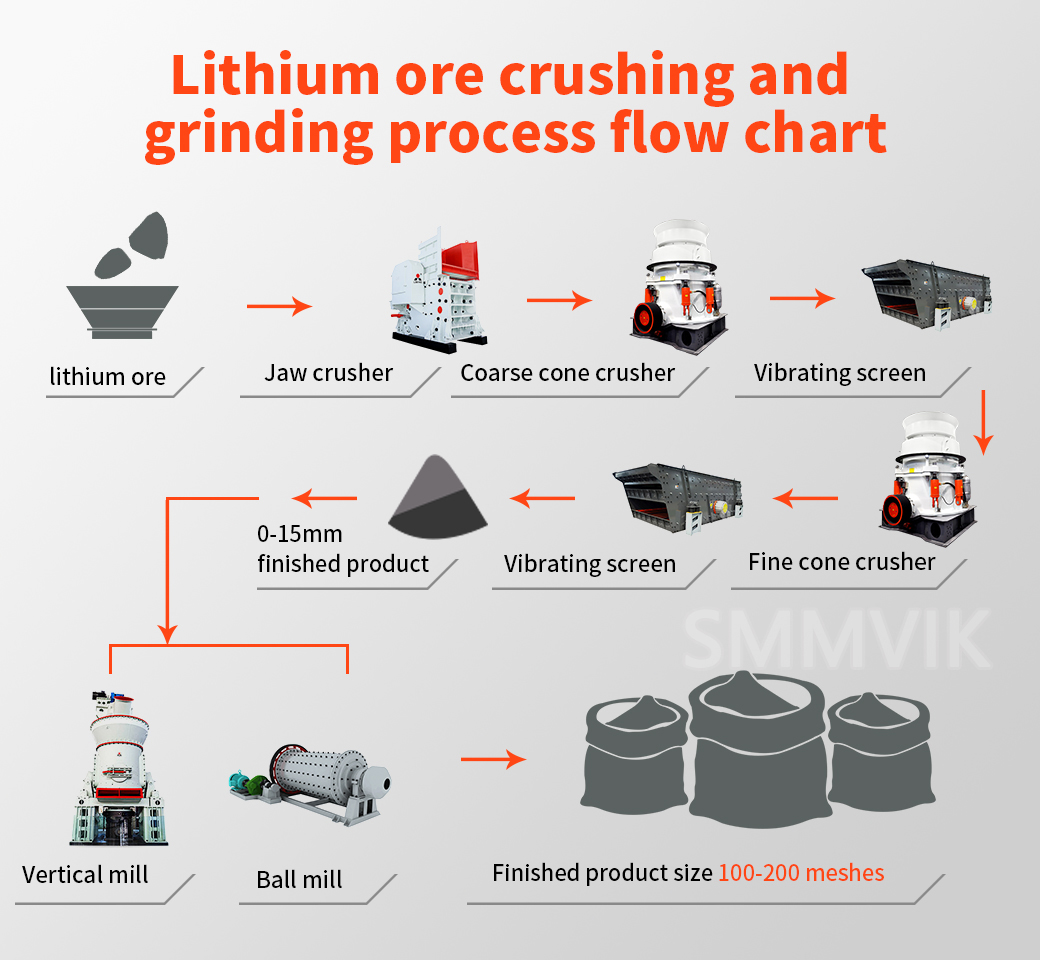
Our series of grinding equipment, such as vertical mill and MTW European version mill, can grind lepidolite and spodumene powder into 80-2500 mesh fine powder, with high grinding efficiency, large output and stable quality of finished products, which can meet the process requirements of lithium carbonate production from lepidolite. If you are interested in our equipment, you are welcome to consult, and we look forward to your arrival.









